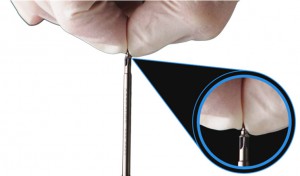
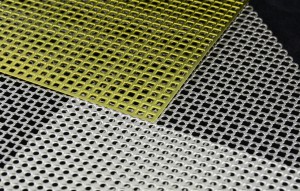
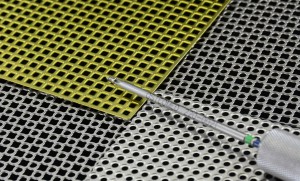
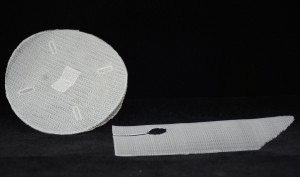
Product Image

MASK SIZE:17.59.5CM
OUTBOX SIZE:59.546*26.5
50pcs/box 4000pcs/ctns
G.W :21kgs
N.W :20KGS
系方式-1024x431.jpg)
Email:inquiry@trulene.net
Tel:+86-13503092791
]]>

Product Standard:《GB19083-2010》
系方式-1024x431.jpg)
Email:inquiry@trulene.net
Tel:+86-13503092791
]]>題-1-1024x1024.jpg)
題-2.jpg)

服-YT-packing1.jpg)

BOX SIZE: 60*41*53
50CPS/CTN
G.W:16KG
系方式-1024x431.jpg)
Email:inquiry@trulene.net
Tel:+86-13503092791
]]>用口罩-1.jpg)
用口罩-2-1024x473.jpg)
用口罩-3-1024x473.jpg)
用口罩-4-1024x473.jpg)
Product Image
Product Standard:《YY/T 0969-2013》
25pcs/Pack , 2Packs/box(50pcs/box)
Box size: 21*10.5*8.5cm
40boxes/CTN(2000PC/CTN)
OUT CARTON SIZE: 44*44*46CM
G.W.:8.84KGS
系方式-1024x431.jpg)
Email:inquiry@trulene.net
Tel:+86-13503092791
]]>

Product Standard:《GB2626-2006》 FFP2/FFP3
Product Specification:5PC/Bag, 1000pcs/CTN
系方式-1024x431.jpg)
Email:inquiry@trulene.net
Tel:+86-13503092791
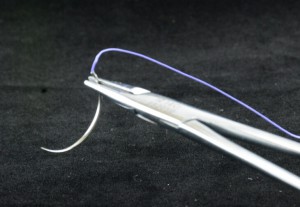
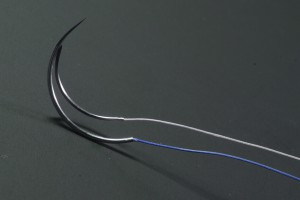
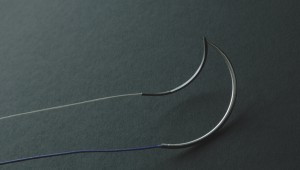
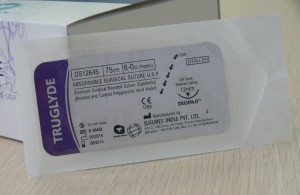
]]>Product standard:Imported product registered standard YZB/IND 1724-2012《Absorbable surgical suture》
Structure and performance:
? ? ? ?Absorbable?surgical suture is composed of needle and the suture, and the needle which is made of stainless steel materials and suture is?a polyglycolic acid, synthetic . D&C Violet?No. 2 dyed to violet?or undyed.
Scope of application:
? ? ?This suture is suitable for the operations that need the artifical?absorbability of surgical suture materials, particularly in the field of intestines and stomach, gynecology and urology and the treatment that need suture and ligation.
]]>Product standard:Import produt register standard YZB/IND 2077-2012《Absorbable Sutures Fast》
Struture and performance:
It is the sterlized?absorbable surgical sutures that made of?100% polyethylene glycol acid. This suture is coated by calcium stearate and polycaprolactone to increase the?lubricating ?performance of suture. Undyed. It has a variety of standard sizes?and lengths, no needle paragraph, or has?various sizes and types of suture with needle.
Scope of application:
Truglyde Fast?applies to those which?need only short-term wound closure support ?or ?need rapidly absorbable suture material that can bring benefits for the soft tissue closure. According to the absorption curve feature of Truglyde Fast , Truglyde Fast is suitable for?skin suture, pediatric surgery, episiotomy, circumcision and mucous membrane suture. Truglyde Fast is not suitable for eye surgery, cardiovascular surgery and neurosurgery.
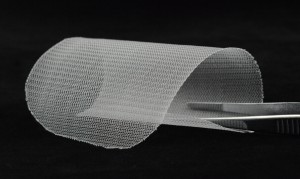
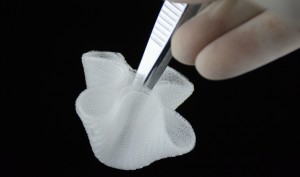
]]>Product Standard:YBZ/IND 4669-2013《non absorbable synthetic surgical mesh》
Structure and performance:
The mesh is made of??braided stretched?monofilament polypropylene , undyed, rectangular and circular. This products is sterilized by ethylene oxide, one-time use.
Scope of application:?
The product is suitable for repairing abdominal wall hernia of the outside abdominal cavity, inguinal hernia, rear abdominal wall hernia, femoral hernia, sciatic?hernia, obturator hernia, perineal hernia, ?and ?abdominal wall defect caused by tumor surgery.
Trulene light polypropylene mesh:
Size: TM611 ? ? ?6*11cm
? ? ? ? ? ? ? TM715 ? ? ?7.5*15cm
? ? ? ? ? ? ? TM151 ? ? ? 15*15cm
? ? ? ? ? ? ? TM303 ? ? ?30*30cm
Self-forming plug:
Size:PL3.8 ? ? ? ?5*10cm ? R=3.8cm

? ?Product standard:?
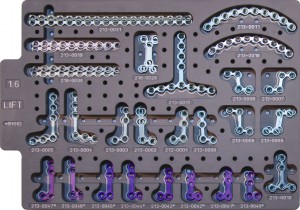
Import product standard YZB/USA 6125-2012《Cranio and Maxillfacial Fixation System》
? ?Struture and performance:
It?is composed of metal plates and metal screw, uses Ti6A14V ELI titanium alloy materials that meets the standard of ASTM F136. Product?surface is without color. Non?sterilization condition.
? ? ?Scope of application:
The product is suitable for cranial and maxillofacial bone trauma or assisting fixation for reconstruction.
2.Cranial and maxillofacial trauma fixation system(product name:OsteoMed CFxTM)
? ? ?Product standard:
Import product register standard YZB/USA 6126-2012 《Cranial and maxillofacial truma fixation system》
?Struture and performance:
The product has metal?plates, titanium mesh and metal screw. Metal screw and partial metal plates is made of Ti6A14V ELI titanium alloy materials according to the standard of ASTM F136, titanium mesh and partial?metal plates is made of pure titanium material ?which meets the standard of?ASTM F67, details as shown in the product size?specification list. Product surface is without color. Non?sterilization condition.
? ? Scope of application:
This product is suitable for the skull fracture,?facial trauma fracture reconstruction.
3.Cranial and maxillofacial plate fixation system(product:OSA System TM)
? ? Product standard:
YZB/USA 6512-2012″Cranial and maxillofacial plate fixation system”
Struture and performance:
Product is composed of metal plates and metal screw, metal plates use grade 2 pure titanium in accordance with ASTM F – 67 , the screw use Ti6A14V ELI titanium alloy materials that meets the standard of ASTM F136 . Product surface is without color. Non?sterilization packaging.
? Scope of application:
This product is applicable to the midface, skull trauma, craniofacial surgery, reconstructive surgery, and the?jaw surgery for upper and lower jaw.
4.Cranial and maxillofacial fixation system ?[M3 trauma Fixation System(Anodized)]
? Product standard:
YZB/USA 0926-2014″Cranial and maxillofacial fixation system”
? Struture and performance:
The production is composed of cranial and maxillofacial fixation plate and screw, the fixed plate is made of grade?1, grade 2, grade 3 and grade 4 pure titanium material that meets the ISO5832-2 standard ; the screw adopt Ti6A14V titanium alloy material that meets the appropriate ISO5832-3 standard, specific list as shown in the specifications, the pure titanium and titanium alloy products surface is treated by anodic oxidation. Non sterilization packaging.
? Scope of application:
Suitable for cranial and maxillofacial fracture internal fixation.
?
5。Titanium mesh【M3 mesh trauma Fixation System(Anodized)]
Product standard:
YZB/USA 0924-2014《Titanium mesh》
Struture and performance:
It is made of grade?1, grade 2, grade 3 and grade 4 pure titanium material that meets the standard of ISO5832-2. ?Details?as shown in the specifications list, the surface is?treated by anodic oxidation .?Non sterilization package.
? ?Scope of application:
Suitable for cranial and maxillofacial fracture internal fixation.
]]>